A child’s first 1,000 days – the period from conception up to two years – are key when it comes to the development of a healthy gut and immune system.1,2
Early life nutrition will enable a healthy immune system and gut microbiota to develop, and specific nutritional components can selectively stimulate the growth and activity of the gut microbiota, thereby impacting the gut microbiota colonization and maturation of the gut and immune system.3
Breastmilk is the best source of nutrition for infants. It supports the development of the gut microbiota and immune system as it contains many nutritional and bioactive compounds such as immune cells, oligosaccharides, and a varying level of bacteria, viable or non-viable, and their metabolites4 that have, most recently, gained more research attention.
The unique composition of breastmilk serves as a model for infant formulae, which seeks to mimic its composition and functionality as closely as possible, through the inclusion of biotics, for infants unable to receive breastmilk: prebiotics, probiotics, synbiotics and the newest member of the family, the postbiotics.
A group of international experts has clarified this emerging concept of postbiotics in a recently published scientific consensus definition in Nature Reviews Gastroenterology & Hepatology (May 2021).5
A new definition
The scientific panel defined a postbiotic as “a preparation of inanimate microorganisms and/or their components that confers a health benefit on the host“.
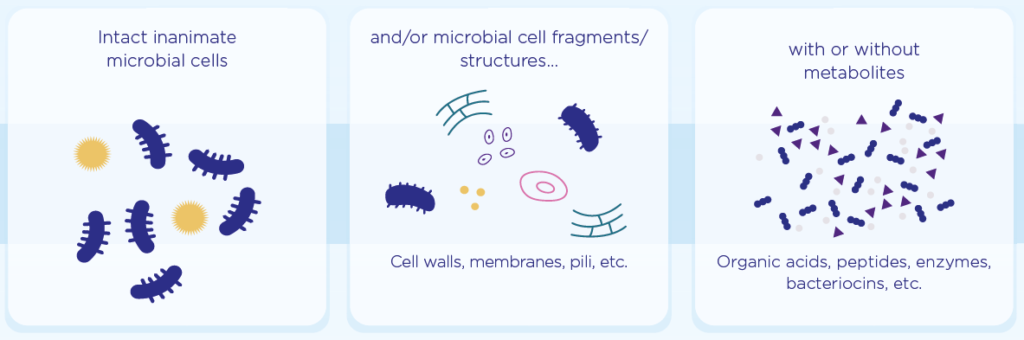
According to the definition:
- A deliberate process to terminate cell viability must be applied.
- The final postbiotic must contain inactivated microbial cells and/or metabolites or cell components.
- A postbiotic does not have to be derived from a probiotic to be accepted as a postbiotic.
- The beneficial effects of a postbiotic on health must be confirmed in the target host.
- Implicit in the definition of a postbiotic is the requirement that the postbiotic is safe for the intended use.
- Postbiotics are likely to be more stable than live counterparts and are less likely to be a safety concern since dead bacteria and yeast are not infective.
WHAT ARE THE SPECIFIC ROLES OF POSTBIOTICS IN THE BODY?
Postbiotics represent an interesting new field of study. Evidence suggests that postbiotics benefits include gut maturity and permeability, reduced severe gastrointestinal infections and strengthening of the immune system6-8.
However, specific benefits will depend on strains and fermentation process used.

To learn more on the biotics family, discover our infography!
DISCOVER HOW OUR UNIQUE POSTBIOTICS ARE PRODUCED!
Go inside Danone Nutricia Research in Utrecht, The Netherlands with more than 50 years of experience in breast milk research.
Visit our ‘fermentation lab’ on floor 1 and watch the video on how our unique postbiotics are created for our infant formula supporting immune system development.
Click here to get the full tour!
References
*ISAPP: The International Scientific Association of Probiotics and Prebiotics
- Godfrey, Keith M., Peter D. Gluckman, and Mark A. Hanson. « Developmental origins of metabolic disease: life course and intergenerational perspectives. » Trends in Endocrinology & Metabolism 21.4 (2010): 199-205.
- Bischoff, Stephan C. « ‘Gut health’: a new objective in medicine?. » BMC medicine 9.1 (2011): 24.
- McKenzie, Craig, et al. « The nutrition‐gut microbiome‐physiology axis and allergic diseases. » Immunological reviews 278.1 (2017): 277-295
- Salminen, Seppo, et al. “Infant formula supplemented with biotics: current knowledge and future perspectives.” Nutrients 12.7 (2020): 1952.
- Salminen, Seppo, et al. “The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics.” Nature Reviews Gastroenterology & Hepatology (2021): 1-19.
- Collado, M. C., G. Vinderola, and S. Salminen. “Postbiotics: facts and open questions. A position paper on the need for a consensus Definition”, Beneficial microbes 10.7 (2019): 711-719.
- Martin, R., et al. « Early life: gut microbiota and immune development in infancy”,Beneficial microbes 1.4 (2010): 367-382
- Mullié, Catherine, et al. “Increased Poliovirus-Specific Intestinal Antibody Response Coincides with Promotion of Bifidobacterium longum-infantis and Bifidobacterium breve in Infants: A Randomized, Double-Blind, Placebo-Controlled Trial”,cPediatric research 56.5 (2004): 791.
BA21-586
